Midterm 1 Study Guide
This is an unofficial study guide I wrote for myself based on the study lists, lectures, and powerpoint slides. Feel free to print for your own use. Notes from the TA Review Session will be in a later post. Check back for updates.
Lecture 1
-
Understand the structural and thermodynamic basis for why hydrolysis of ATP releases a lot of free energy. Know why ATP hydrolysis releases more energy than hydrolysis of glycerol-3-phosphate.
1) Hydrolysis of ATP releases a lot of free energy because it releases phophate, which is a very good leaving group (it is resonance stabilized). 2) Hydrolysis releaves charge repulsion on ATP. 3) The product ADP is quickly ionized, thereby removing a product. 4) ADP + Pi are more hydrated than ATP.
ATP hydrolysis cleaves an anhydride bond, whereas hydrolysis of glycerol-3-P cleaved an ester bond. Anhydride hydrolysis is more thermodynamically favorable than ester hydrolysis because the products of anhydride cleavage are more resonance stabilized (anhydrides have two carboxyl groups, whereas an ester only has one carboxyl group attached to an O-CH3.)
- Know why Pi is a good leaving group. Resonance stabilization.
- Know ATP structure and the nomenclature of the phosphate groups on ATP (α, β, γ)
-
Know two types of bonding of the phosphates in ATP (anhydride and ester)

The alpha phosphate is attached to the ring by an ester bond, whereas the beta and gamma phosphates have anhydride bonds.
-
Understand why hydrolysis of anhydride is more favorable than hydrolysis of an ester
Cleavage of an anhydride produces two molecules that are resonance stabilized with carboxyl groups. Cleavage of an ester bond produces one molecule with a carboxyl group, and one molecule without.
-
Know why PEP, Phosphocreatine, and 1,3-Bisphosphoglycerate are high-energy compounds (no need to memorize the structures of the 3 compounds, but you should understand the chemical processes. For example, you do not need to know how to draw PEP, but you should know Pi is a good leaving group and the enol form of pyruvate can be spontaneously converted to the keto form of Pyruvate).
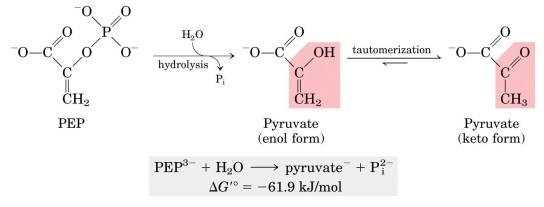
PEP is a high energy compund because Pi is a good leaving group, and tautomerization of the enol product to the keto form effectively removes the product, driving the rxn forward. The hydrolysis of Phosphocreatine removes Pi, and produces a resonance stabilized product (Creatine) which is also a good leaving group. 1,3-Bisphosphoglycerate hydrolysis removes Pi, and the product is subsequently ionized, producing a final product which is resonance stabilized.
-
You should be able to explain whether hydrolysis of any phosphorylated compounds is favorable or not based on the principles we covered in this lecture
Lecture 2
-
Understand the relationship between ∆G, ∆H, and ∆S.
∆G = ∆H – T ∆S
-
Know the relationship between ∆G, spontaneity, and equilibrium.
∆G <0 spontaneous
∆G = 0 at equilibrium
∆G >0 non-spontaneous
-
Know the relationship between ∆G and reactants/products concentrations
∆G = ∆G’0 + RT ln([A]/[B])
-
Know how to calculate equilibrium constants from ∆G’0 or from Keq to ∆G’0.
ΔG’° = -RTlnKeq
-
Understand the meaning of reaction coordinate curve ( For example, know where is ∆G on the curve) and know how to draw reaction curve if ∆G values are given.

-
Coupling reactions: know thermodynamic basis for coupling reactions (know why free energy changes are additive, you also should know why small free energy change can lead to a large keq change).
In coupling rxns, energy released from one reaction is used to help drive the other. For the reactions 1) A –> B and 2) B –> C, the B’s cancel out and we are left with the rxn 3) A –> C. The free energy changes for 1 and 2 can be added to get the free energy change of 3. keq1 = [B]/[A], keq2 = [C]/[B], and keq3 = ([B][C])/([A][B]). So while the free energy changes are additive, the keq is multiplicative. Therefore, a small free energy change can lead to a large keq change.
-
Understand whether ATP hydrolysis can drive thermodynamically unfavorable reactions.
ATP can drive thermodynamically unfavorable reactions, but not simply by hydrolysis. First, ATP –> ADP, creating a good leaving group (the Pi in glutamyl phosphate). Then nucleophilic attack is much more favorable.

-
Understand whether ATP hydrolysis can shift equilibrium of other reactions.
For an unfavorable reaction A–>B, we know that the ΔG is positive (say, +4.0 Kcal/mol). With ATP hydrolysis, ΔG becomes a negative number (say, -3.3 Kcal/mol). If we plug the original (+4.0) ΔG into this equation: keq = [B]eq/[A]eq = 10^ΔG/1.36, we get a very small equilibrium ratio (1.15×10^-3). If we plug in the (-3.3) ΔG with ATP hydrolysis, we will find that keq is MUCH larger (1.34×10^5). So yes, ATP hydrolysis and change the equilibrium ratio, thereby shifting the equilibrium of an unfavorable reaction.
-
Understand why ATP-driving reactions are not simply hydrolysis ATP, instead phosphorylated intermediates are formed. You should know the example we described in the lecture.
See example above.
-
You should know the consequences if a nucleophile attacks different phosphates on ATP and why difficult reactions such as activation of fatty acids are driven by adenylation.

In adenylation (c), the R group is attached to a larger leaving group: P-Rib-Adenine. Larger leaving groups are easier to remove, so nucleophilic attack (in the subsequent step) is more favorable.
-
You are required to know the details of firefly and luciferase.
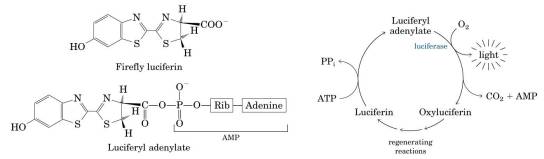
-
Thioester bond HsCoA: understand why hydrolysis of a thio-ester bond releases more energy than hydrolysis of a normal oxygen ester bond.
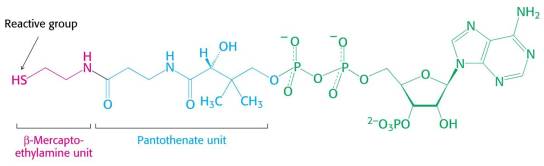
Coenzyme A (CoA)

The oxygen ester bond is more stable than the thioester bond because it is more stabilized by resonance (shown above). Also, S is bigger than O, which draws more electrons away from the carbonyl carbon. This greater partial positive charge on the thioester carbonyl carbon makes it easier to be hydrolysed.
-
Understand what is the electron donor and acceptor when glucose is converted to CO2 and water.
C6H12O6 + 6O2 -–> 6CO2(-2) + 6H2O(-2)
Oxidation: C6H12O6 –> 6CO2
Reduction: 6O2 –> 6H2O
So, glucose is the e- donor, O2 is the e- acceptor.
-
Truly understand where the electrons come from when an organic molecule is oxidized.
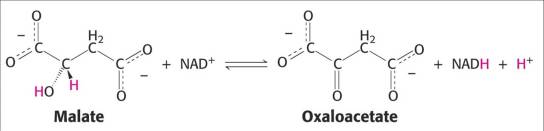
-
Understand the difference between NAD + and NADP+
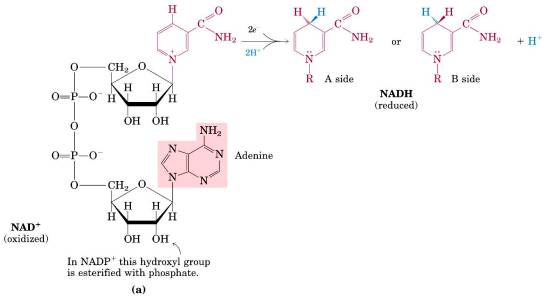
-
Understand the different roles of NADH and NADPH in catabolism and anabolism
NADH is usually used in catabolism (“breaking down”), while NADPH is used in anabolism (biosynthesis, “building up”).
Lecture 3
-
Know what’s the reduced form of FAD and FMN

-
Understand why FAD/FMN can do both one electron and two-electron chemistry
Because they can either be partially reduced to the radical (semiquinone) form, or fully reduced (see figure above).
-
Understand why Coenzyme Q can do both one and two-electron chemistry

-
Know why cytochromes can only do one-electron chemistry

Example: Fe(+3) + e- <–> Fe(+2)
-
You should recognize the electron carriers, but you are NOT required to draw the structures.
-
Understand the meaning of “dehydrogenase”
A dehydrogenase is an enzyme that reduces a substrate by transferring one or more protons and a pair of electrons to an acceptor, like NAD/NADP or FAD/FMN.
-
Know what’s electronegativity and how to use it to determine relative oxidation/reduction state. You should be able to know which compounds are the most reduced and which ones are the most oxidized if I give you a list of compounds.

-
You should know the relative energy levels of organic molecules. For example, you should be able to predict which compounds can release more energy when they are completely oxidized to carbon dioxide if I give you a list of compounds.
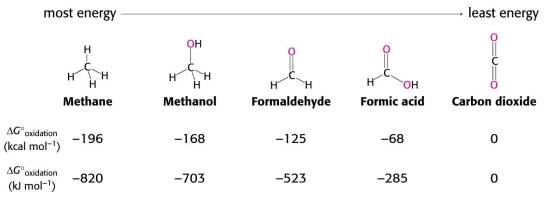
-
Know what are redox potentials and the usefulness of the redox potentials. For example, you should know which ones would function as a reducing agent and which ones normally function as oxidizing agents if I tell you the redox potentials.
The redox potential (E’0 = standard reduction potential) is a measure of the tendency of a system to donate or accept electrons. Reducing agents donate electrons and have a negative redox potential, while oxidizing agents accept electrons and have a positive redox potential.
-
Understand how standard redox potential is measured
Using a salt bridge with a reference cell and a test cell.
-
Understand the relationship between E and E’0.
Spont. = ΔE = Eacceptor – Edonor
E’0 is measured under standard conditions (25degreesC, 1M concentrations, 1atm)
-
Know the relationships between ΔG and ΔE, ΔG’0 and ΔE’0, ΔE’0 and Keq. Know how to calculate one term if the other terms are given.
ΔG = -nFΔE where n = # e-‘s
ΔG = -RTlnKeq
so, ΔE = (RTlnKeq)/nF
-
Know the relationship between ΔE and spontaneity.
The greater (more positive) the ΔE, the more spontaneous the reaction is and visa versa.
-
Know how to calculate ΔE if E’0 and concentrations of reactants and products are given.
E = E’0 + (RT)/(nF) ln([e- acceptor]/[e- donor])
-
Understand the general mechanisms of isomerization, addition/elimination reactions.

isomerization
group transfer

hydrolysis

addition-elimination
Lecture 4
-
Know how to indicate ΔG, ΔG‡, in the reaction diagram.
See reaction diagram from Lecture 2 (above).
-
Know the implications of ΔG and ΔG‡ (ΔG determines spontaneity and ΔG‡ determines rate constants)
ΔG shows whether or not the product has lower free energy than the starting material (if ΔG is negative, then the reaction is spontaneous because the product is at a lower energy level). ΔG‡ shows the relationship between the starting material and the activation energy: it tells us the rate at which the reaction can go forward.
-
Know the effects of enzymes/catalysts on ΔG, and ΔG‡
ΔG is unaffected by enzymes/catalysts, while ΔG‡ is lowered. Enzymes change the pathway from S–>P, but the start and finish remain the same.
-
Know how to draw reaction diagram of enzyme-catalyzed reactions and know the difference between enzymecatalyzed and non-catalyzed reactions.

-
Know the 4 types of strategies used by enzymes to accelerate reactions
1) Enzymes provide a different pathway from S–>P.
2) They decrease the ΔG‡.
3) They facilitate the formation of the transition state.
4) They stabilize the transition state by specific interactions between the enzyme and the transition state molecule.
-
Understand why intra-molecular reactions are usually faster than inter-molecular reactions.
Intra-molecular reactions are usually faster because the two interacting parts of the molecule are “tied” together, thereby increasing the odds of their interaction. In inter-molecular reactions, two separate molecules are interacting, and the odds of their reacting with one another are much less because they are free to move around. Intra-molecular reactions which have non-flexible bonds have an even greater chance of reacting.
-
Know the relationship between reaction rate constant and the activation energy (you need to memorize the equation)
k = Ae^-ΔG‡/(RT)
-
You need to understand the consequences of activation energy decrease or increase on reaction rate constants in a quantitative way (again, you should know the equation and how to use the equation to solve problems)
The rate constant k will decrease with increased activation energy, and visa versa.
-
Understand why enzymes can accelerate reactions.
See above.
-
Know the relationship between ΔG‡ and Keq if there is a relationship.
There is a relationship between Keq and ΔG (ΔG’0 = -RTlnKeq), but the activation energy does NOT change Keq. Enzymes accelerate the forward and reverse reactions to the same extent.
-
No need to memorize the reaction catalyzed by citrate synthase for now. We will discuss the reaction more in Krebs cycle
-
Understand covalent catalysis and nucleophilic catalysis. You should know why formation of a covalent intermediate could speed up reactions.

covalent catalysis
The formation of a transient covalent bond can speed up the reaction by stabilizing the transition state between the intermediate (A) and the product (B).

nucleophilic catalysis
In nucleophilic catalysis, the nucleophile forms an intermediate which is more easily broken down to products.
-
Understand acid and base catalysis and know the difference between specific and general acid/base catalysis.
Acid makes attack easier by protonation of the molecule to be attacked. Protonation also makes better leaving groups. In specific acid-base catalysis, protonation occurs before attack by water. You need a strong acid in specific acid-base catalysis in order for full protonation to occur. In general acid-base catalysis, the proton is transferred during the slow step, and a weak acid is sufficient.
-
Understand why metal-ions can do catalysis. Know the three different mechanisms a metal-ion can participate in catalysis.

A) Metal ions can increase the susceptibility of nucleophilic attack by increasing the difference in charge.
B) They can make the leaving group a weaker base (weak bases are better leaving groups).
C) Metal can bind to water, which increases the nucleophilicity of the hydroxide ion.
-
Know the single letter code for the 20 amino acids
G – Glycine (Gly) P – Proline (Pro) A – Alanine (Ala) V – Valine (Val)
L – Leucine (Leu) I – Isoleucine (Ile) M – Methionine (Met) C – Cysteine (Cys)
F – Phenylalanine (Phe) Y – Tyrosine (Tyr) W – Tryptophan (Trp) H – Histidine (His)
K – Lysine (Lys) R – Arginine (Arg) Q – Glutamine (Gln) N – Asparagine (Asn)
E – Glutamic Acid (Glu) D – Aspartic Acid (Asp) S – Serine (Ser) T – Threonine (Thr)
-
Primary structure, secondary structure, tertiary structure, quaternary structure.

Beta sheets consist of beta strands that are connected laterally by H-bonds. They are twisted in an alpha helical structure.
Lecture 5
-
Know how to derive the Michaelis-Menten equation and understand the underlying assumptions.
V0 = (Vmax[S])/(Km + [S])
DERIVATION:
E + S <–>(k1/k-1) ES –>(k2) E + P
Reason we can ignore backward rxn (k-2) for last step: [P] ~ 0, so we can ignore it
Formation of ES: k1[E][S]
Decomposition of ES: k-1[ES] + k2[ES]
k1[E][S] = k-1[ES] + k2[ES]
[S] >> [E]
[S] = [S]0, so we can ignore the change in [S]
[E] ≠ [E]0
[E] = [E]0 – [ES]
substitution: k1([E]0 – [ES])[S] = k-1[ES] + k2[ES]
k1[E]0[S] – k1[ES][S] = k-1[ES] + k2[ES]
k1[E]0[S] = k1[ES][S] + k-1[ES] + k2[ES]
[ES] = (k1[E]0[S])/(k1[S] + k-1 + k2) = ([E]0[S])/([S] + ((k-1 + k2)/k1))
V0 = k2[ES] = (k2[E]0[S])/([S] + ((k-1 + k2)/k1)) = (Vmax[S])/([S] + km)
since km = (k-1 + k2)/k1
-
Know the definition of Vmax and Km and their relationship with k1, k2, and k-1
for E + S <–>(k1/k-1) ES –>(k2) E + P,
Vmax = k2[E]0 km = (k-1 + k2)/k1
-
Know where are the Km and Vmax when you are given a plot with Vo as the Y axis and [S] as the X-axis
Vmax is the upper limit of the curve. km = 1/2Vmax
-
Know the slope and intercepts on Y and X-axis when you plot (1/V0) against (1/[S])

-
Know what is a kcat and its relationship with other rate constants
kcat is the rate constant for a catalysed reaction. kcat = k2, so Vmax = kcat[E]0.
-
Is kcat a constant? Is Vmax a constant? Is Km a constant?
kcat is a constant. Vmax is not a constant because it depends on the initial enzyme concentration. Km is a constant.
-
What is the difference between Km and Kd? What is the relationship between km and substrate affinity?
kd is the dissociation constant: the greater it is, the less affinity it has for a substrate. kd is equal to (k-1/k1) because it describes the binding step (E + S <–> ES). km, on the other hand, takes into acount the rate constants for the entire reaction (E + S <–> ES –> E + P). Since km = k-1/k1 + k2/k1, km = kd + k2/k1. So when k2 << k-1 (k2 is rate-limiting), then we can ignore k2 and km = kd. For most enzymes, however, this is not the case.
-
Understand the general strategies to regulate metabolism (enzyme concentrations, enzyme activity, and substrate accessibility)
1) Enzyme concentrations: transcription, translation, degradation 2) Enzyme activity: allosteric regulation, covalent modification, proteolytic activation 3) Substrate accessibility: compartmentalization
-
Understand the concept of allosteric regulation. Understand why a molecule that is totally different from an enzyme substrate in structure can regulate the enzyme activity.
In allosteric regulation, activation or inhibition of the enzyme is controlled by an effector which binds to the enzyme at a different site than the substrate. This binding of the effector causes a conformational change in the enzyme which either increases or decreases its affinity for the substrate. Has an s-shaped kinetic curve.
-
No need to memorize the mechanism of ATCase I presented in the class.
-
You need to know what is a protein kinase and what kind reactions a kinase can do, what kind substrates and products are involved in a kinase-catalyzed reactions.
A protein kinase is an enzyme which catalyzes the phosphorylation of specific amino acid residues of a protein. Kinases in general are involved in phosphorylation: the transfer of phosphate groups from a high energy compound (like ATP) to a substrate. For example a hexokinase transfers a phosphoryl group from ATP to glucose (6-C = hexo).
-
Understand what is a phosphatase, what kind substrates and products are involved in a protein phosphatase reaction.
A phosphatase is the opposite: it REMOVES a phosphoryl group from its substrate. The product is a molecule with a free hydroxyl group + phosphate ion.
-
Understand why phosphorylation/dephosphorylation is an effective way to regulate metabolism.
1) Electrostatic (charge) interactions can be disrupted or formed (a phosphorylated protein carries a -2 charge) 2) A phosphate can form 3 or more H-bonds 3) Link energy laws to regulation 4) Signal amplification: kinase cascade
-
Understand why both the kinase and phosphatase catalyzed reactions are essentially irreversible

Pi is a good leaving group.
Lecture 6
-
Know all the reactions in glycolysis including structures of each intermediate, and enzymes discussed in the lecture.


-
You should be able to draw structures of every intermediate in glycolysis
-
You should be able to trace any labeled carbon in glucose in any glycolysis intermediates. For example, if carbon 6 in glucose is labeled with C14, you should be able to identify where is the C14 in Pyruvate or any other intermediates.
-
Know which part is the preparatory phase and which part is the payoff phase
-
Understand why ATP has to be consumed during the preparatory phase, and how ATP is generated during the payoff phase
It takes energy input to make energy. Glucose has to be primed with phosphate groups which come from two molecules of ATP. The resulting two molecules of ADP are later phosphorylated in the payoff phase to form two molecules of ATP. In the last step, two more molecules of ADP are converted to two more molecules of ATP. Net: 2 ATP.
-
Understand the roles of phosphorylation of intermediates (transport, binding, ATP synthesis)
-
Understand why the first step of glycolysis is the formation of G-6-P, and not G-1-P or G-2-P.
-
Why does G-6-P have to be converted to Fructose-6-P and how the conversion occurs (you need to know the isomerization of G-6-P is via the linear structures)?

-
Is the second phosphorylation necessary for splitting glucose to two 3-carbon molecules?

-
Understand what kind reactions an aldolase can do.
Aldolases are involved in reversible aldol condensation reactions, in which a C-C bond is formed (or cleaved) by the nucleophilic addition of an enolate to an aldehyde to form a beta-hydroxy ketone (aldol).
-
Understand whether glucose can be cut by aldolases and if so, what kind products will be generated.
-
Understand why it is better to split hexose to two 3-carbon molecules than to generate one 4-carbon and one 2carbon molecule.
-
Understand the roles of the second phosphorylation since the step is not necessary for splitting the molecule.
-
Again, it is important for you to understand the material, not simply memorize the pathway.
-
Understand the triose phosphate isomerase (general acid/base catalysis and an enol intermediate). Know what is the common feature between triose isomerization and glucose isomerization to fructose (They both involve an enol intermediate)
WOW! Um, can i sit by you in class? hahaha i’m kidding. kind of.
katharrine
January 21, 2010
student of university
jalil
March 23, 2010
you are amazing!
Carolin, Erin, Kate or Lexy
January 21, 2011
do you happen to have the study guide answers for midterm 2? =] THANKS
kljdfg
January 26, 2011
Thank you! I hope you were able to find the information you needed. Keep checking back since I am planning to resurrect the entire blog… (I haven’t been posting since Winter ’09 since that is when I graduated, but I have plans for the blog to go “2.0” in the near future.)
Mary
March 1, 2011
Thank you for your comment. Unfortunately, I got really busy during the last half of that course and I never got around to posting the second study guide. I have since graduated and haven’t posted since, but stay tuned because I have plans to resurrect the blog and make it “2.0”, or user-submitted content. I am beginning to work on that now, so hopefully it will be up and running with new content possibly in time for next quarter!
Mary
March 1, 2011